OBJECTIVE: To understand some important features of subatomic particles.
|
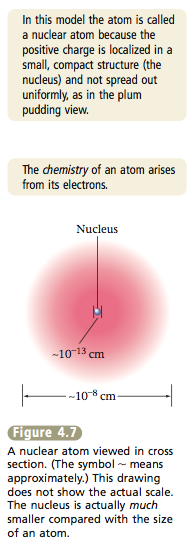
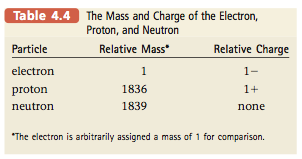
The space in which the electrons move accounts for most of the atomic volume. The electrons are the parts of atoms that “intermingle” when atoms combine to form molecules. Therefore, the number of electrons a given atom possesses greatly affects the way it can interact with other atoms. As a result, atoms of different elements, which have different numbers of electrons, show different chemical behavior. Although the atoms of different elements also differ in their numbers of protons, it is the number of electrons that really determines chemical behavior.
|